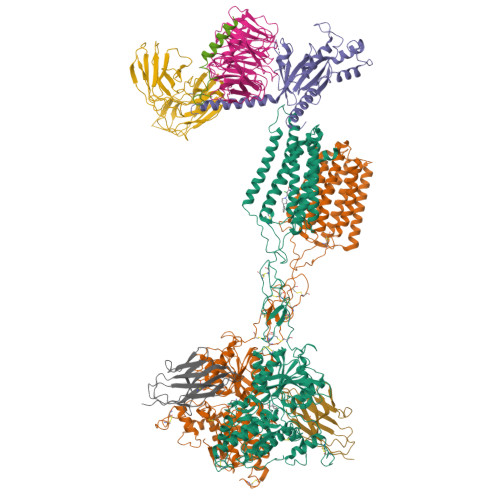
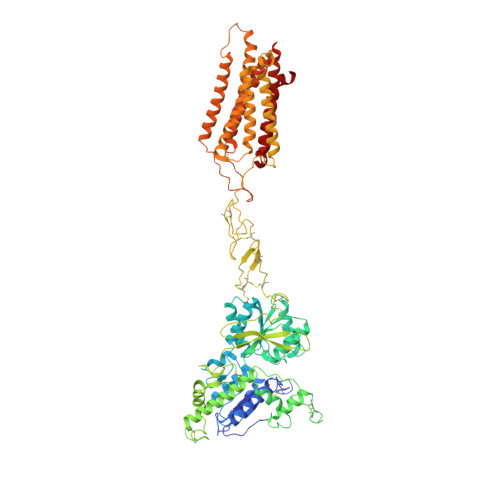
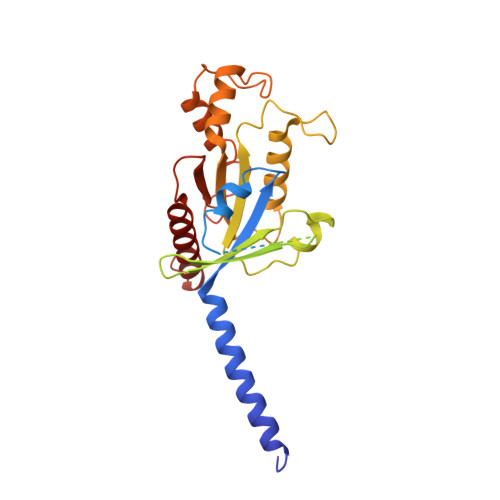
Structures of G i -bound metabotropic glutamate receptors mGlu2 and mGlu4.
Lin, S., Han, S., Cai, X., Tan, Q., Zhou, K., Wang, D., Wang, X., Du, J., Yi, C., Chu, X., Dai, A., Zhou, Y., Chen, Y., Zhou, Y., Liu, H., Liu, J., Yang, D., Wang, M.W., Zhao, Q., Wu, B.(2021) Nature 594: 583-588
- PubMed: 34135510
- DOI: https://doi.org/10.1038/s41586-021-03495-2
- Primary Citation of Related Structures:
7E9G, 7E9H - PubMed Abstract:
The metabotropic glutamate receptors (mGlus) have key roles in modulating cell excitability and synaptic transmission in response to glutamate (the main excitatory neurotransmitter in the central nervous system) 1 . It has previously been suggested that only one receptor subunit within an mGlu homodimer is responsible for coupling to G protein during receptor activation 2 . However, the molecular mechanism that underlies the asymmetric signalling of mGlus remains unknown. Here we report two cryo-electron microscopy structures of human mGlu2 and mGlu4 bound to heterotrimeric G i protein. The structures reveal a G-protein-binding site formed by three intracellular loops and helices III and IV that is distinct from the corresponding binding site in all of the other G-protein-coupled receptor (GPCR) structures. Furthermore, we observed an asymmetric dimer interface of the transmembrane domain of the receptor in the two mGlu-G i structures. We confirmed that the asymmetric dimerization is crucial for receptor activation, which was supported by functional data; this dimerization may provide a molecular basis for the asymmetric signal transduction of mGlus. These findings offer insights into receptor signalling of class C GPCRs.
Organizational Affiliation:
CAS Key Laboratory of Receptor Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, China.